Europe style for FDA approved Test Kit - Elisa Test Kit of AMOZ – kwinbon
Europe style for FDA approved Test Kit - Elisa Test Kit of AMOZ – kwinbon Detail:
2.The nitrofuran drugs furaltadone, nitrofurantoin and nitrofurazone were banned from use in food animal production in the EU in 1993, and the use of furazolidone was prohibited in 1995. The analysis of nitrofuran drugs residue needs to be based on the detection of the tissue bound metabolites of the nitrofuran parent drugs, since the parent drugs are very rapidly metabolized, and the tissue bound nitrofuran metabolites will retain for a long time, therefore the metabolites are used as the target in the detection of the abuse of nitrofurans. Furazolidone metabolite (AMOZ), Furaltadone metabolite (AMOZ), Nitrofurantoin metabolite (AHD) and Nitrofurazone metabolite (SEM).
Details
1.Elisa Test Kit of AMOZ
2.Cat. KA00205H-96 Wells
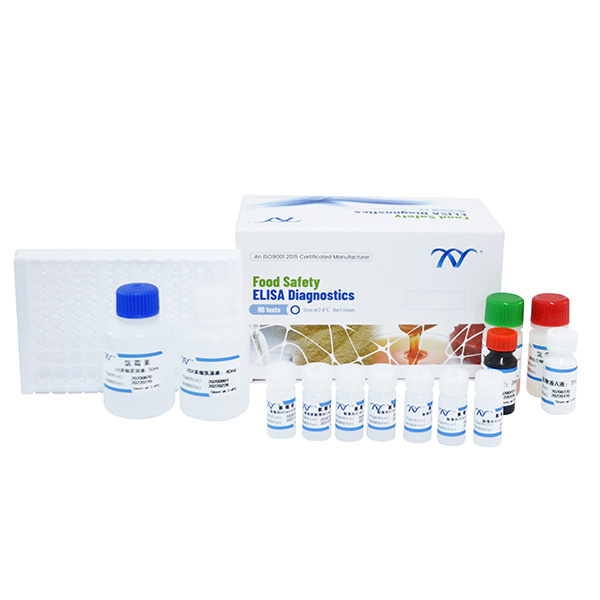
3.Kit Components
● Microtiter plate with 96 wells coated with antigen
● Standard solutions(6 bottles)
0ppb, 0.05ppb,0.15ppb,0.45ppb,1.35ppb,4.05ppb
● Spiking standard solution: (1ml/bottle) ……………………………………………...100ppb
● Enzyme conjugate 1ml…………………………………………………..…………….red cap
● Antibody solution 7ml ………………………………………………..………….….green cap
● solution A 7ml…………………………………………………….…………………white cap
● solution B 7ml………………………………………………………….………..…… red cap
● stop solution 7ml ……………………………………………………….…………yellow cap
● 20×concentrated wash solution 40ml…………………………………….……transparent cap
● 2×concentrated extraction solution 50ml……………………………………….…….blue cap
● 2-Nitrobenzaldehyde 15.1mg………………………………………………….……white cap
4.Sensitivity, accuracy and precision
Sensitivity: 0.05ppb
Detection limit
Aquatic products(fish and shrimp)………….……… 0.1ppb
Accuracy
Aquatic products(fish and shrimp)….…...………… 95±25%
Precision: CV of the ELISA kit is less than 10%.
5.Cross Rate
Furaltadone metabolite(AMOZ)………………………………….…………100%
Furazolidone metabolite(AMOZ)…………………………..………………..<0.1%
Nitrofurantoin metabolite(AHD)……………………………….……………<0.1%
Nitrofurazone metabolite(SEM)…………………………………………..…<0.1%
Furaltadone…………………………………………………………….…….11.1%
Furazolidone……………………………………………………….….…..…<0.1%
Nitrofurantoin……………………………………………………….…….…<1%
Nitrofurazone…………………………………………….………………..…<1%
Product detail pictures:

Related Product Guide:
We offer great strength in quality and development,merchandising,sales and marketing and operation for Europe style for FDA approved Test Kit - Elisa Test Kit of AMOZ – kwinbon , The product will supply to all over the world, such as: Borussia Dortmund, Holland, Cancun, Our team knows well the market demands in different countries, and is capable of supplying suitable quality products at the best prices to different markets. Our company has already set up a professional, creative and responsible team to develop clients with the multi-win principle.
Although we are a small company, we are also respected. Reliable quality, sincere service and good credit, we are honored to be able to work with you!