Europe style for FDA approved Test Kit - Isoprocarb Residue Detection Test Card – kwinbon
Europe style for FDA approved Test Kit - Isoprocarb Residue Detection Test Card – kwinbon Detail:
Pesticide properties for Isoprocarb, including approvals, environmental fate, eco-toxicity and human health issues.
Details
Isoprocarb Residue Detection Test Card
Cat. KB11301K-10T
About
This kit is suitable for qualitative detection of residual isoprocarb in fresh cucumber sample.
Isoprocarb is a touch-and-kill, quick-acting pesticide, which is a highly toxic pesticide. It is mainly used to control rice planthopper, rice cicada and other pests on rice, some fruit trees and crops. Toxic for bees and fish.
High performance liquid chromatography-tandem mass spectrometry was used for residue determination because of high selectivity and simple treatment. Compared with HPLC methods, our kit show considerable advantages regarding sensitivity, detection limit, technical equipment and time requirement.
Sample preparations
(1)Before testing, the samples should be restored to room temperature (20-30℃).
Fresh samples should be taken to wipe away the soil and cut into pieces less than 1cm square.
(2) Weigh 1.00± 0.05g sample into 15mL polystyrene centrifuge tube, then add 8mL extract, close the lid, oscillate up and down manually for 30s, and let it stand for 1min. Supernatant liquid is the sample to be tested.
Note: The sampling method refers to the food safety sampling inspection administration measures (aqsiq decree no. 15 of 2019). GB2763 2019 for reference.
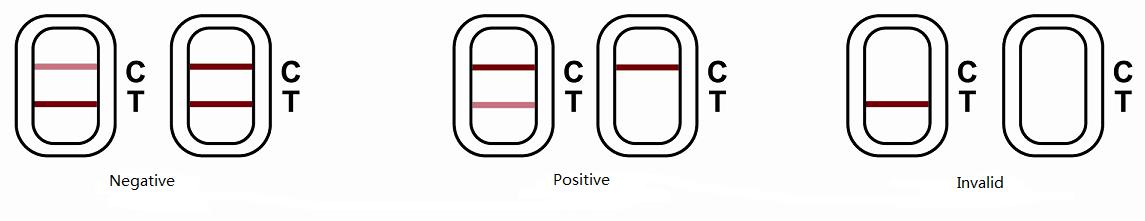
Results
Negative(-) : Line T and Line C are both red, color of Line T is deeper than or similar to Line C, indicating the isoprocarb in sample is less than LOD of the kit.
Positive(+) : Line C is red, color of line T is weaker than line C, indicating isoprocarbl in sample is higher than LOD of the kit.
Invalid: Line C has no color, which indicates the strips are invalid. In this case, please read the instructions again, and redo the assay with new strip.
Storage
Save the kits in a dry environment of 2 ~ 30℃ away from light.
The kits will be valid in 12 months.
Product detail pictures:

Related Product Guide:
We've got a specialist, effectiveness staff to supply high quality service for our shopper. We always follow the tenet of customer-oriented, details-focused for Europe style for FDA approved Test Kit - Isoprocarb Residue Detection Test Card – kwinbon , The product will supply to all over the world, such as: Algeria, Georgia, New Zealand, With the principle of win-win, we hope to help you make more profits in the market. An opportunity is not to be caught, but to be created. Any trading companies or distributors from any countries are welcomed.
The sales person is professional and responsible, warm and polite, we had a pleasant conversation and no language barriers on communication.